Aptamer Group plc (LON:APTA), the leading developer of next-generation synthetic binders delivering innovation to the life science industry, has announced a new development and licensing agreement with an established global company in the life sciences and diagnostics sector, part of a major multinational conglomerate.
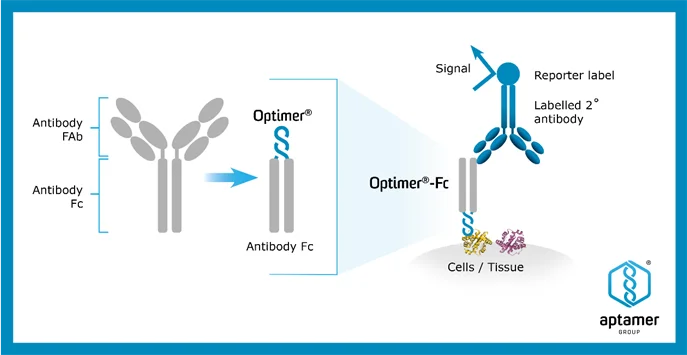
Under the agreement, Aptamer will develop a custom Optimer®-Fc reagent for use in immunohistochemistry (IHC) assays and diagnostic kits, leveraging its proprietary Optimer®-Fc platform, which produces aptamer binders with an antibody Fc-tag optimised for IHC workflows. The Partner will fully fund the development work, which is expected to be completed within a 12-month timeline, for an undisclosed sum. Subject to development and validation, the Optimer®-Fc reagent is anticipated to be incorporated into commercial IHC kits, with a product launch expected within the current calendar year.
The agreement includes a commercial licensing arrangement, under which Aptamer will receive royalties of 2% on net sales of all assay kits containing the licensed Optimer®-Fc reagent, subject to standard minimum royalty commitments. The kits are expected to be distributed through recognised global channels specialising in life sciences research products.
This partnership strengthens Aptamer’s recurring revenue potential and supports its strategy to expand the application of its Optimer platform in high-value life sciences markets. The collaboration with a globally respected industry leader underscores the commercial potential and technical capabilities of Aptamer’s Optimer®-Fc technology for advanced diagnostic applications.
Dr Arron Tolley, Chief Executive Officer of Aptamer Group, commented:
“We are pleased to collaborate with a leading global life sciences company to develop this Optimer®-Fc reagent for IHC applications. The royalty structure and fully funded development provide a clear path to revenue generation and validate the strength of our Optimer platform. This agreement enhances our portfolio of royalty-generating assets and supports our mission to deliver innovative solutions to the life sciences industry.”
Certain details of the agreement, including specific financial terms and the identity of the Partner, are commercially sensitive and have not been disclosed at this stage. The Company will provide updates on material developments as appropriate.