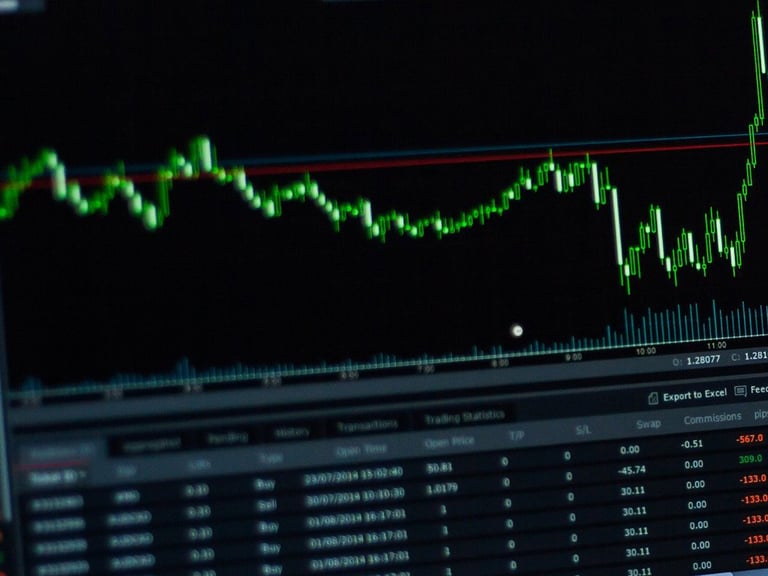
GSK plc which can be found using ticker (GSK) now have 4 confirmed analysts covering the stock with the consensus suggesting a rating of ‘Hold’. The target price High/Low ranges between 45 and 35.5 and has a mean target at $39.67. Now with the previous closing price of $36.65 this now indicates there is a potential upside of 8.2%. Also worth taking note is the 50 day moving average now sits at $35.52 and the 200 day moving average is $34.85. The company has a market cap of $75,572m. Find out more information at: https://www.gsk.com
The potential market cap would be $81,799m based on the market consensus.
GSK plc, together with its subsidiaries, engages in the research, development and manufacture of vaccines and specialty medicines to prevent and treat disease in the United Kingdom, the United States, and internationally. It operates through four segments: Pharmaceuticals, Pharmaceuticals R&D, Vaccines, and Consumer Healthcare. The company offers pharmaceutical products comprising medicines in the therapeutic areas, such as infectious disease, HIV, immunology and respiratory, and oncology. The company was formerly known as GlaxoSmithKline plc and changed its name to GSK plc in May 2022. GSK plc was founded in 1715 and is based in Brentford, the United Kingdom.
The company has a dividend yield of 4.01% with the ex dividend date set at 23-2-2023 (DMY).
Other points of data to note are a P/E ratio of 13.57, revenue per share of 14.57 and a 6.57% return on assets.