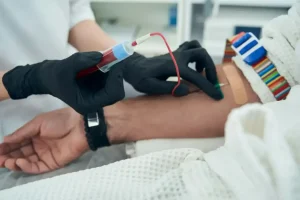
EDX Medical Group Plc (AQSE:EDX) is a global specialist in digital clinical diagnostics; developing and supporting high-performance products and services to provide cost-effective prediction of disease risk, inform clinical decision-making, enable personalised healthcare and accelerate the development of new medicines for cancer, neurology, heart disease and infectious diseases.
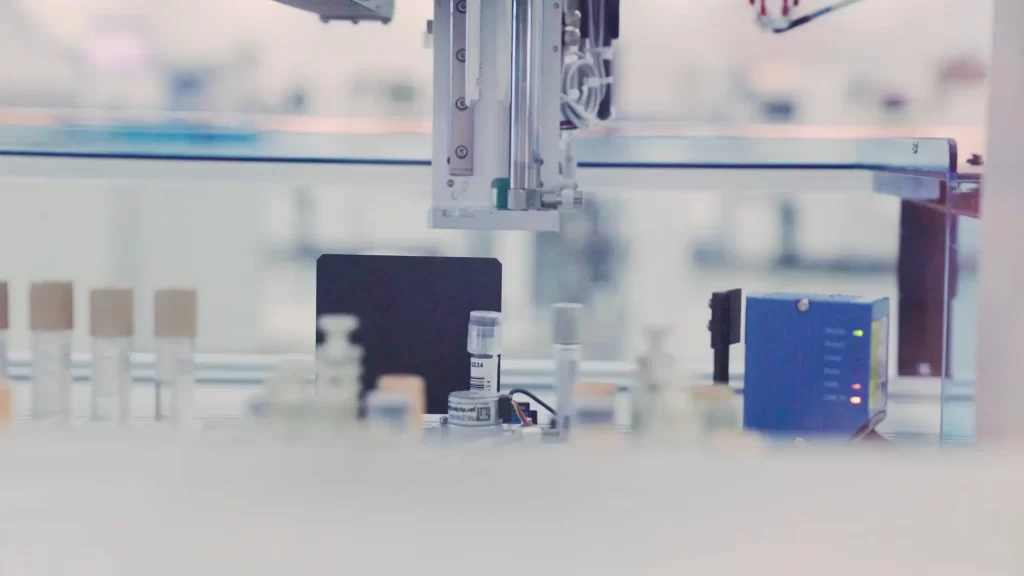
EDX Medical’s trusted diagnostic solutions combine outstanding biological and digital technologies.
These digitally-enabled products and services can set new standards in risk assessment, personal diagnosis and enable better clinical decision-making.
In addition to advanced laboratory tests, their scientists in Cambridge and Oxford, UK, are in the late stages of developing a range of tests capable of accurately measuring a combination of multiple disease markers on a single device within minutes.
These tests will be used to identify early signs of cancer, infections, and cardio-vascular conditions and can be carried out by health professionals at the point-of-care, wherever they work, without delays and the costs associated with laboratory processing.
EDX MEDICAL GROUP PARTNERS:


EDX Medical Group has entered into a collaborative agreement with Thermo Fisher Scientific EMEA Ltd, a world leader in supplying life sciences solutions and services.
The collaboration agreement will enable EDX Medical and Thermo Fisher to jointly develop and potentially commercialise a number of clinical-grade assays – including novel and innovative cancer diagnostic solutions.
The multi project collaboration will harness Thermo Fisher’s powerful technologies and information translation systems to deliver a number of advanced testing solutions in development at EDX or licensed from partners, including a proprietary assay to enable personalised radiotherapy and a novel chemotherapy toxicity assay focusing on serious adverse events.
EDX Medical entered into a strategic agreement with Guardant Health Inc., under which they distribute:
- Guardant360® CDx, a blood test that provides doctors with tumour mutation profiling, for solid cancers, and
- Guardant Reveal™, a blood-only liquid biopsy test that detects circulating tumour DNA (ctDNA) for minimal residual disease (MRD) assessment in colorectal, breast, and lung cancer,
to the private healthcare market in the UK and to the public and private sectors in Sweden, Denmark, Norway, Finland and Iceland (Nordics).